
DermaSensor: The device that is revolutionizing skin cancer detection
Reserve today for $199 and SAVE $200 off list price ($399)
Not ready to sign up? Speak to a sales representative

DermaSensor: The device that is revolutionizing skin cancer detection
Reserve today for $199 and save $200 off list price ($399)
Not ready to sign up? Speak to a sales representative
DermaSensor May Help Your Patients
- Avoid unnecessary biopsies
- Avoid additional visits to a dermatologist, along with the associated out-of-pocket expenses
- Feel more confident in your management of questionable lesions
DermaSensor May Help You
- Make better and more confident decision
- Increase your reimbursement level by augmenting your typical skin assessment routine
- Increase revenue by introducing a value-based skin health service with out-of-pocket contributions by patients
- Improve your practice’s image by incorporating new and innovative technology

Make DermaSensor™ Your New Assistant For Skin Cancer Care
We’ve launched the world’s first point-and-click device that brings objective skin cancer assessment to primary care offices. You now have a tool to help evaluate patient skin cancer risk in just seconds.
- Rapid, non-invasive skin cancer assessment by any trained team member
- Objective result can improve your clinical decisions and confidence
- Improve your skin cancer detection by 13% as demonstrated in a study with 57 GPs
- Can help avoid benign excisions with unnecessary morbidity and poor reimbursement
Rapid Recording
Quick, point-and-click recordings collect and analyze non-visual spectral data quickly
Optical Spectroscopy
Light-emitting tip non-invasively scans the lesion, receiving and analyzing spectral data at the cellular level from below the skin's surface
Easy to Use
Wireless, ergonomic design makes the device intuitive to learn and easy to use
Immediate Result
DermaSensor's proprietary algorithm provides an instant, objective result to support your clinical decision at the point of care
*Minimum commitment $199+GST one-time Account Activation Fee + $79+GST for 10 patients per month usage plan

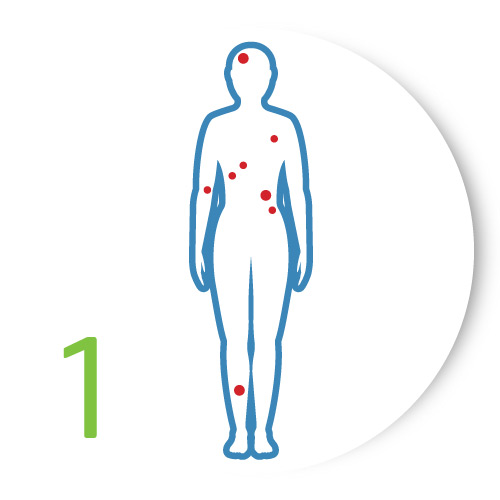
Assess
Assess the patient's lesions to identify those visually suggestive of skin cancer for which an objective risk assessment would be helpful.
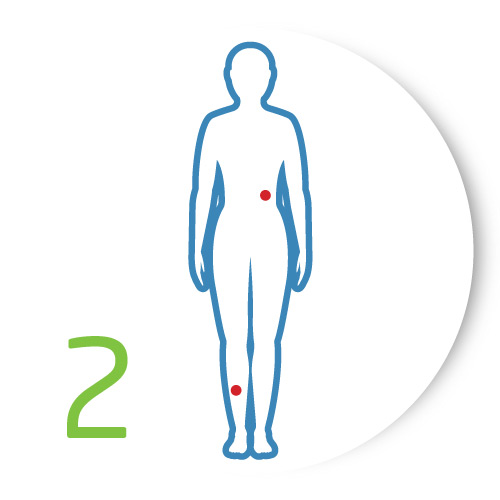
Analyze
Use DermaSensor to analyze those lesions of concern to receive an "Investigate Further" or "Monitor" result for each lesion
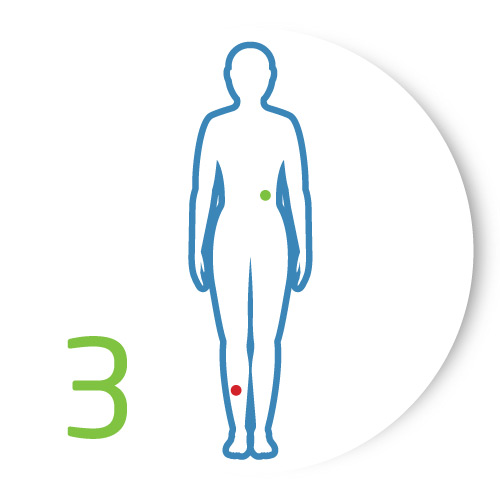
Act
Counsel your patient on next steps, such as further investigating the lesion through a biopsy or specialist referral, or just monitoring the lesion having ruled out the need for an immediate biopsy or referral.

Simple Design.
Effortless Application.
- Intuitive on-screen user interface guides you step-by-step through the evaluation
- Activate a recording using either the touch-screen interface or conveniently positioned side buttonsDermaSensor is not a camera or dermatoscope. DermaSensor uses pulses of light and elastic scattering spectroscopy to assess a lesion’s cellular and sub-cellular structures.
DermaSensor is not a camera or dermatoscope. DermaSensor uses pulses of light and elastic scattering spectroscopy to assess a lesion’s cellular and sub-cellular structures.
Performance in clinical validation studies:
All clinical validation studies reported no adverse events, and studies showed DermaSensor sensitivity superior to GPs and similar to Dermatologists.1,3,4
For non-melanoma skin cancer (NMSC), i.e. BCC and SCC, DermaSensor’s performance was 88-98% based on dermatopathology.1,3,4
88-97%
DermaSensor’s spectroscopy technology showed a 88-97% sensitivity for melanoma (including highly atypical nevi) in three multi-year, multi-site prospective studies.1,3,4
94%

Skin cancer detection results improved from 81% to 94% with use of the DermaSensor device.2
Risks
False-positive and false-negative results may lead to unnecessary care or to a malignant skin lesion not being optimally managed, respectively. However, it is important to note that biopsy is used to confirm pathology and that elastic scattering spectroscopy is to be used as an adjunctive tool to visual inspection and history-taking. See all Claims and Substantiations
DISCLAIMER: The DermaSensor Device is currently CE Marked and is available for sale in Australia.
FDA submission pending review; not available for sale in the United States.
80-0010.1
1Manolakos D, Rabinovitz H, Geisse J, Bonning M, Rodriguez-Diaz E, Bigio I, Cognetta A. Clinical Validation of a Handheld Elastic Scattering Spectroscopy-Artificial Intelligence Device. Presentation, AAD Innovations Academy, July 21-24, 2022.
2Tepedino K, Tablada A, Barnes E, Da Silva, T. Clinical Utility of a Handheld Elastic Scattering Spectroscopy Tool and Machine Learning on the Diagnosis and Management of Skin Cancer by Primary Care Physicians. Poster Presentation, SDPA Fall Conference, Nov 4-7, 2021.
3Hartman, R. Tepedino, K., Fung, MA., McNiff, JM., Grant-Kels, J. Clinical Validation of a Handheld Elastic Scattering Spectroscopic Device in the Evaluation of Lesions Suggestive of Melanoma, Presentation at the American Academy of Dermatologists Annual Meeting, Mar 24-28th, 2022.
4Data on file, DermaSensor, Inc.